STRUCTURE AND BONDING: atomic orbitals, ionic bonding, covalent bonding and electronic configuration in organic chemistry
What is organic chemistry? The answer is all around. The proteins that make up our hair, skin, and muscles; the nucleic acids, RNA and DANA, that control our genetic heritage; the foods we eat; the clothes we wear; and the medicines we take, all are organic chemicals.
The foundation of organic chemistry we're built in the mid-eighteenth century as Chemistry was evolving from an alchemist's art into a modern science. At that time, unexplained differences we're noted between substances derived from living sources and those derived from minerals. Compounds from plants and animals were often difficult to isolate and purify. Even when pure, these compounds were difficult to work and were more sensitive to decomposition than compounds from mineral sources. In 1770, the swedish chemist Torbern Bergman first expressed this difference between "organic" and "inorganic" substances, and the phrase organic chemistry soon came to mean the Chemistry of compounds from living organisms.
To many chemist at that time, the only explanation for the difference in behavior between organic and inorganic compounds was that organic compounds contained a peculiar and u definable "vital force" as a result of their coming from living sources. One consequence of the presence of this vital force, chemist believed, was that organic compounds could not be prepared and manipulated in the laboratory as could inorganic compounds.
Although the vitalistic theory was believe but many influential chemist, it's acceptance was by no means universal, and it's doubtful that the development of organic chemistry was much delayed. As early as 1816, the theory received a heavy blow when Michel chevreul found that soap, prepared by the reaction of alkali with animal fat, could be separated into several pure organic compounds, which he termed "fatty acids". Thus, for the first time, one organic substance (fat) had been converted into others (fatty acids plus glycerin) without the intervention of an outside vital force.
 |
| hydrolysis of soap to produce fatty acids |
A little more than a decade later, the vitalistic theory suffered still further when Friedrich Wohler discovered I. 1828 that it was possible to convert the "inorganic" salt of ammonium cyanate into the previous known "organic" substance urea.
 |
| wholer synthesis of urea from ammonium cyanate |
By the mid-nineteenth century, the weight of evidence was clearly against the vitalistic theory. In 1848, William Brand wrote in a paper that "No definite line could be drawn between organic and inorganic chemistry, any distinctions must for the present be merely considered as matters of practical convenience calculated to further the progress of students. " Chemistry today is unified; that same basic scientific principle that explains the simplest inorganic compounds also explains the most complex organic molecules. The only distinguishing characteristics of organic substance is that all contain the elememt carbon. Nevertheless, the division between organic and inorganic chemistry, which began for historical reason, maintains its "practical convenience to further the progress of students."
Organic chemistry, then, is the study of carban compounds. Carbon which has atomi number 6, is a second-period element whose position in an abbreviated periodic table is shown below. Although carbon is the principal elememt in organic compounds, most also contain hydrogen, and many contain Nitrogen, oxygen, phosphorus, sulphur, chlorine, and other elements.
 |
| an abbreviated periodic table |
Uniqueness of carbon
Why is carbon special? What is it that sets carbon apart from other elements in the periodic table? The answers to this questions are complex but has to do with the following;- carbon lies at the centre of the second period in the periodic table between electron donors (left) and electron acceptors (right). It therefore readily shares electrons to form millions of covalent compounds
- Has half filled shell in the second period which confers some degree of stability.
- it forms long chains of repeated units (catenation) and various sizes of ring structures.
- It forms single, double, and triple bonds with itself and other atoms.
The Nature of Atoms: Quantum Mechanics
Throughout the nineteenth century, and into the twentieth, scientists sought to understand the nature of the atom and the nature of the forces holding atoms together in molecules. A major breakthrough occured 1926 when the theory of quantum mechanics was proposal independently by Paul Dirac, Werner Heisenberg, and Erwin Schrodinger. All three formulation are mathematical expressions that describes the electronic structure of atoms, but Schrodinger's is the one most commonly used by chemists.The Schrodinger equation offers a delailed description of the electronic structure of atoms. It says that the motion of an electron around the nucleus can be described mathematically by what's known as a wave equation, the same kind of equation that's used to describe the motion of waves in fluid.
The solution to a wave equation is called a wave function. If we could determine the wave function for every electron in an atom, we would have a complete electronic description of that atom. In practice, wave equations are mathematically so complex that only approximate solutions can be obtained, even with the fastest computers now available. This approximate solution agree so well with experimental facts, however, that quantum mechanics is a universally accepted theory for understanding atomic structure.
The Nature of Atoms: Atomic Orbitals
How can we interpret quantum mechanical wave functions in terms of physical reality? A good way of viewing a wave function is to think of it as an expression that predicts the volume of space around a nucleus where an electron can be found. Though we can never know the exact position of an electron at a given moment, the wave function tells us where we would be most likely to find it.The volume of space around a nucleus in which an electron is most likely to be found is called an orbital. It's often helpfull to think of an orbital as a kind of time-lapse photograph of an electron's movement around the nucleus. Such a photograph will show the orbatal as a blurry cloud indicating where the orbital has previously been. This electron cloud doesn't have a descrete boundary, but for practical purposes we can set the limits of an orbital by saying that it represents the space where an electron spends most (90-95%) of it's time.
What do orbital look like? The exact shape and size of an electrons orbital depends on it's Energy level. Electron can be thought of as belonging to different layers, or shell, around the nucleus, where each she'll contains different numbers and kinds of orbitals. For example, the first shell (the one nearest the nucleus) has only one orbital, called a 1s orbital. The second second shell has four Orbitals, 2s and three 2p; the third shell has nine orbitals, one 3s, three 3p, five 3d and so on. Relative energy level of the different kinds of atomic Orbitals are shown below;
 |
| Relative energy of atomic orbitals |
The lowest-energy electrons occupy the 1s orbital. The s atomic Orbitals are spherical and have the nucleus of the atom at their centre, as shown in fig 1.0 below. Next in energy after the 1s electrons are the 2s electrons. Because they are higher in energy, 2s electrons are farther from the positively charged nucleus on average, and their spherical orbital is somewhat larger than that of 1s electrons.
The 2p electrons are next higher in energy, as shown in fig 1.1 below. It indicates there are three 2p Orbitals, each of which is roughly dumbell shaped. The three 2p Orbitals are equal in energy and are oriented in space such that each is perpendicular to the other two. They are denoted 2px, 2py, and 2pz, to show on which axis they lie. Note that the plane passing between the two lobes of a p orbital is region of zero electron density. This nodal plane has certain consequences with respect to chemical bonding.
 |
| the spherical shape of an s atomic orbital |
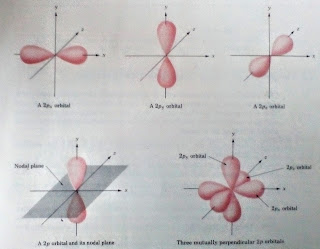 |
| Shapes of 2p orbitals |
 |
| A 3d orbital |
The Nature of atoms: electronic configuration
The lowest-energy arrangement, or ground state electronic configuration, of an atom is a description of what orbitals the atom''s electrons occupy. This arrangement can be found by using our knowledge of atomic Orbitals and their energy levels. Available electrons are assigned to the proper Orbitals by following the rules:Always fill the lowest energy Orbitals first (called the aufbau principle).
Only two electrons can be put into each orbital, and they must be of opposite spin ( called the Pauli exclusion principle).
If two or more empty orbital of equal energy are available, put one electron in each until all are half-full before pairing ( called Hund's rule).
Let's look at some examples to see how this rules are applied. Hydrogen, the lightest eelment, has only one electron, which we assign to the lowest energy Orbital. This gives hydrogen a 1s ground-state configuration. Carbon has 6 electrons, and a ground state configuration 1s2 2s2 2px 2py is arrived by applying the three rules. These are other examples as shown in the table below;
 |
| Ground state electronic configuration of some elements |
Problem exercise:
Problem 1.0
Give the ground state electronic. Configuration for these elements
(a) Boron (b) phosphorus (c) Oxygen (d) Chlorine.
Problem 2.0
How many electrons does each of these elements have I. It's outermost electron shell?
(a) potassium (b) Aluminium (c) Krypton
Development of chemical bonding theory
By the mid-nineteenth century, with the vitalistic theory of organic chemistry dead and with the distinction between organic and inorganic chemistry nearly gone, chemist began to probe the forces holding molecules together. in 1858, August Kekule and Archibald couper independently proposed that, in all organic compounds, carbon always has four "affinity units". that is carbon is tetravalent; it always form four bonds when it joins other elements to form compounds. Furthermore, said Kekule, carbon atoms can bond to each other to form extended chains of atoms linked together.
shortly after the tetravalent nature of carbon was proposed, extensions to the Kekule - couper theory were made when the possibility of multiple bonding between atoms was suggested. Emil Erlenmeyer proposed a carbon to carbon triple bond for acetylene. in 1865, Kekule provided another major advance in bonding theory when he postulated that carbon chains can double back on themselves to form rings of atoms.
Perhaps the most significant early advance in understanding bonding in organic molecules was the contribution made independently by jacobus van't Hoff and Joseph Le Bel. Although Kekule had satisfactorily described the tetravalent nature of carbon, chemistry was viewed in an essentially two-dimensional way until 1874. in that year, van't Hoff and Le Bel added a third dimension to our conception of molecules by proposing that the four bonds of carbon have specific spatial direction. van't Hoff even went further and correctly proposed that the four atoms to which carbon is bonded sit at the corners of a tetrahedron, with carbon in the center. A representation of a tetrahedral carbon atom is shown below.
Note carefully the conventions used in the figure below to show three-dimensionality.:
Heavy wedged lines represents bonds coming out of the page toward the viewer;
Normal lines represent bonds in the plane of the page and;
Dashed lines represents bonds receding back behind the page, away from the viewer.
 |
| tetrahedral carbon atom |
Problem 3.0:
Draw a Molecule of chloroform, CHCL3, using wedged, normal, and dashed lines to show its tetrahedral geometry.
Chemical bonding: Ionic bonds
what is the modern picture of chemical bonding? why do atoms bond together, and how does the quantum-mechanical view of the atom describe bonding? the why question is relatively easy to answer: Atoms form bonds because the substance that results is more stable (has less energy) than the alternative arrangement of isolated atoms. Energy is always released when a chemical bond is formed. The how question is more difficult. To answer this, we need to know more about the properties of atoms.
We know through emperical observation that eight electrons (an octet) in the outermost electron shell impacts certain stability to the inert gas elements in group 0 (or 8): Ne (2+8); Ar(2+8+8); Kr (2+8+18+8). We also know that the chemistry of many elements with nearly inert gas configurations is dominated by attempt to achieve the stable inert-gas electronic makeup. the alkali metal in group 1 for example, have single s electrons in their valence shell. By losing this electron, they can achieve an inert-gas configuration.
The amount of energy it takes to pull an electron away from an atom is called the ionization energy (IE) of the element. Alkali metals, at the far left of the periodic table, give up an electron easily, have low ionization energies, and are thus said to be electropositive. Elements at the middle and far right of the periodic table hold their electrons more tightly, give them up less readily, and therefore have higher ionization energies (IE's). In other words, a low ionization energy correspond to the ready loss of an electron, and a high ionization energy correspond to the difficult loss of an electron.
Atom + Energy (IE) ------------- uni-positive atom + electron
Just as the electropositive alkali metals at the left of the periodic table have the tendency to form positive ions by losing an electron.
The halogens (group VIIA elements) at the right of the periodic table have a tendency to form negative ions by gaining an electron. By so doing, the halogens can achieve an inert -gas configuration. The measure of this tendency to gain an electron is called the Electron Affinity (EA). energy is released when an electron is added to most elements, and electron affinities are therefore negative numbers.
Elements on the right of the periodic table have a much greater tendency to add an electron than elements on the left side and are said to be electronegative. Thus, the haloges release a large amount of energy when they react with an electron and have much larger negative electron affinities than the alkali metals.
Atom + electron ---------------- uni-negative atom + energy (EA)
 |
| electron affinities of some elements |
The simplest kind of chemical bonding is that between an electropositive element (low IE) and electronegative element (large negative EA). For example, when sodium metal [ IE = 118 kcal/mol (494 kj/mol)] reacts with chlorine gas [ EA= -83.2 kcal/mol (- 348kj/mol)], sodium donates an electron to chlorine ions. The product, sodium chloride, is said to have ionic bonding. That is, the ions are held together purely by electrostatic attraction between the two unlike charges. A similar situation exists for many other metal salts such as potassium fluoride, lithium bromide, and so on. This picture of the ionic bond first proposed by Walter Kossel in 1916, satisfactorily accounts for the chemistry of many inorganic compounds.
Chemical bonding: covalent bonds
Element on the left and right side of the periodic table form ionic bonds by gaining or lossing an electron to achieve an inert-gas configuration. How, though, do elements in the middle of the periodic table form bonds? Lets look at the carbon atom in methane, CH4, as an example. Certainly the bonding in methane isn't ionic, since it would be very difficult for carbon either to gain or lose four electrons to achieve an inert-gas configuration. In fact, carbon bonds to other atoms, not by donating electrons, but by sharing them. Such shared-electron bonds, first proposed in 1916 by G.N. Lewis, are called covalent bonds. The covalent bond is the most important bond in organic chemistry.
A simple shorthand way of indicating covalent bonds in molecules is to use what is known as Lewis Structures, or electron-dot structures. In this method, the outer-shell electrons of an atom are represented by dots. Thus, hydrogen has one dot representing its 1s electron, carbon has four dots , oxygen has six dots, and so on. A stable molecule results whenever the inert-gas configuration is achieved for all atoms, as in the following examples;
Lewis structure are valuable because they make electron "bookkeeping " possible and constantly remind us of the outer-shell electrons (valence electrons) involved. Simpler still is the use of "Kekule" structure, also called line-bond structure, in which a two-electron covalent bond is indicated simply by a line drawn between atoms. Pairs of unbonding valence electrons are often ignored when drawing line-bond structures, but you must still be mentally aware of their existence . It's useful when starting out always to include them. Some of the molecules already considered are shown below;




0 Comments