50 CHEMISTRY QUESTIONS AND ANSWERS FOR POST UTME STUDENTS INVOLVING ELECTROLYSIS, SOLUBILITY, ORGANIC, NUCLEAR, EMPERICAL FORMULA ETC
1. What is the molecular formula of the compound whose empirical formula is CH2O and molar mass is 180? (H=1, C=12, O=16):(A)C6H12O6 (B)C4H8O5 (C) C6H10O5 (D) C4H8O2.
2. Which of the pollutant is biodegradable? (A) Plastics (B) Sewage compounds (C) Metal scraps (D) Hydrogen sulphide.
3. Which of the following equations represents the reaction leading to the removal of permanent hardness of water?(A) MgSO4 + Na2CO3=MgCO3 + Na3SO4 (B) Ca(OH)2 + 2HCl=Cacl2 + 2H2O (C) Ca(HCO3)2 + Ca(OH2)=2CaCO3 + 2H2O (D) MgSO4 + Bacl2=Mgcl2 + BaSO4.
4. How many moles of AgNO3 are there in 500cm3 of 0.01 M AgNO3 solution? (A) 0.005 mol (B) 0.05 mol (C) 0.5 mol (D) 1.0 mol.
5. Which of the following statements explains why tetraoxosulphate (vi) acid is regarded as a strong acid? (A) The acid is basic (B) the acid is concentrated (C) the acid is completely ionized in aqueous solution. (D) tetraoxosulphate (vi) ions are very reactive.
6. To what temperature must a gas be raised from 273k in order to double both its volume and pressure? (A) 300K (B) 546K (C) 819K (D) 1092K.
7. If 3 moles of electrons are required to deposit 1 mole of a metal, M during the electrolysis of its molten chloride, the empirical formula of the metallic chloride is? (A) M3CL (B) M3CL2 (C) M2CL3 (D) MCL3.
8. Nuclear reactions can be used in the following except: (A) gauging the thickness of object (B) making atomic bombs (C) curing cancer (D) purifying water.
9. Which of the following compound crystallizes without water of crystallization? (A) Na2CO3 (B) CuSO4 (C) MgSO4 (D) NaCL.
10. The product of the electrolysis of dilute sodium chloride solution with platinium electrodes are? (A) oxygen and clorine (B) chlorine and water (C) hydrogen and oxygen (D) sodium amalgam and chlorine.
11. Which of the following statement is not correct of group seven elements? (A) they are diatomic (B) they are good oxidizing agent (C) they are highly electronegative (D) they have relatively low ionization energy.
12. Which of the following statement is not correct of cathode ray? (A) are positive charged (B) travel in straight line (C) are deflected away from negative plates.
13. CH4g + 2O2g=2H2O + CO2g H = 890kj/mol, H in the reaction represented by the equation is called the enthalpy of : (A) formation (B) combustion (C) activation (D) neutralization.
14. Compounds that have the same molecular formula but different structures are said to be : (A) isomeric (B) isotopic (C) polymeric (D) allotropic.
15. When a crystal is added to its solution, it did not dissolve and the solution remained unchanged, showing that the solution was: (A) concentrated (B) unsaturated (C) colloidal (D) saturated
16. When steam is passed over white hot coke, the products are: (A) carbon (iv) oxide and nitrogen (B) carbon (ii) oxide and hydrogen (C) carbon (ii) oxide and nitrogen (D) carbon (iv) oxide and hydrogen.
17. The maximum number of electrons that can be accommodated in the shell having the principal quantum number 3 is : (A) 3 (B) 9 (C) 18 (D) 32.
18. Methanol is obtained from wood by (A) esterification (B) destructive distillation (C) combustion (D) fractional distillation.
19. Study carefully the reaction represented by the equation: 2H2O2= O2g + 2H2Og. which of the following will not increase the reaction rate? (A) Heating the hydrogen peroxide (B) adding a pinch of MnO2 to the reactant (C) increase the concentration of H2O2. (D) Adding water to the reactant.
20. Which of the following process is a physical reaction? (A) Electrolysis (B) Hydrolysis (C) Allotropic change (D) Neutralization.
21. The following acids are monobasic except: (A) methanoic acid (B) dioxonitrate(iii) acid (C) ethanedioic acid. (D) oxochlorate (i) acid.
22. The rate of reaction is proportional to the number of effective solutions occurring per second between the reactants this statement is associated with the : (A) kinetic theory (B) rate law (C) atomic theory (D) Collision theory.
23. In the reaction represented by the following equation; 2H2Sg + SO2g 2H2Ol + 3Sg SO2 is acting as: (A) a reducing agent (B) an oxidizing agent (C) a dehydrating agent (D) a bleaching agent.
24. When iron rust, it undergoes: (A) chemical decomposition (B) hydrolysis (C) redox reaction (D) combustion
25. The following salts are readily dissolve in water except: (A) Na2CO3 (B) Pb(NO3)2 (C) Kcl (D) FeSO4.
26. When sucrose is warmed with fehlings solution: (A) a silver mirror is produced (B) solutions turns milky (C) brick-red precipitate is formed (D) there is ho precipitate.
27. The ionic radii of metals are usually : (A) greater than their atomic radii (B) unaffected by the charge on the ion (C) less than their atomic radii (D) greater than those of non-metals.
28. Which of the following compounds is not a raw material for the manufacture of plastics? (A) Ethane (B) ethene (C) monochloroethene (D) propene.
29. The energy required to remove the most loosely bound electron from an atom in the gaseous state is known as: (A) bond energy (B) ionization energy (C) potential energy (D) activation energy
30. If a reaction is said to be exothermic, which of the following statements is a correct deduction from the information? (A) The reaction vessel gets hotter as the reaction proceeds/ (B) H for the reaction is positive. (C) The rate of reaction increases with time. (D) The activation energy of the reaction is high.
31. Which of the following PH values is likely to be that of a slightly alkaline solution? (A) 2 (B) 5 (C) 7 (D) 8.
32. Which of the following minerals contains fluorine as one of its constituent elements? (A) cryolite (B) Bauxite (C) potash alum (D) kaolin.
33. The product of the rection between propanoic acid and ethanol is: (A) ethylpropanoate (B) ethylethanoate (C) methylpropanoate (D) propylethanoate.
34. Which of the following accounts for the difference in the mode of conduction of electricity by metals and aqueous salt solution? (A) Electrons are present in metals but not in salt solution. (B) Metals are conductor while salts are electrolyte (C) electricity is carried by mobile electrons in metals but by ions in aqueous salt solution. (D) salts ionizes in aqueous solution while metals do not.
35. Starch undergoes complete hydrolysis to produce: (A) maltose (B) lactose (C) fructose (D) glucose.
36. Which of the following solids has network structure? (A) Diamond (B) iodine (C) sulphur (D) graphite.
37. The properties of electrovalent compounds include the following except: (A) high melting and boiling point (B) conduction of electricity in the molten state (C) high volatility at room temperature (D) ionization in aqueous solution.
38. Which of the following pairs illustrate isotopy? (A) but-1-ene and but-2-ene (B) carbon and hydrogen (C) oxygen and ozone (D) hydrogen and deuterium.
39. Carbon is often deposited in the exhaust-pipe of cars because of the: (A) presence of carbon in petrol (B) dehydrogenation of petrol (C) incomplete combustion of petrol (D) presence of additives in petrol.
40. Sulphur burns in air to form: (A) a basic oxide (B) an acidic oxide (C) an amphoteric oxide (D) a neutral oxide.
41. Chlorine is used in water treatment as: (A) germicide (B) a decolorizing agent (C) an antioxidant (D) a coagulating agent.
42. What amount of copper will be deposited if a current of 10A was passed through a solution of copper (ii) salt for 965seconds? (1F= 96500c): (A) 0.005 mol (B) 0.025 mol (C) 0.05 mol (D) 1.00 mol.
43. What volume of distilled water should be added to 400cm3 of 2.0 mol/dm3 H2SO4, to obtain 0.2 mol/dm3 of solution? (A) 600cm3 (B) 800cm3 (C) 1000cm3 (D) 4000cm3.
44. The component of air that is removed when air is bubbled through alkaline pyrogallol solution is: (A) carbon (iv) oxide (B) oxygen (C) water vapour (D) nitrogen.
45. An alkene can be converted to an alkane by: (A) halogenations (B) hydrolysis (C) dehydration (D) hydrogenation.
46. The product of the reaction between ethanol and excess acidified K2Cr2 O7 is; (A) ethanol (B) ethylethanoate (C) ethanoic acid (D) ethyne.
47. How many faradays of electricity are required to liberate 9g of aluminum? (Al= 27) (A) 0.1 (B) 0.3 (C) 1.0 (D) 3.0
48. Alums are classified as: (A) simple salts (B) acid salts (C) anhydrous salts (D) double salts.
49. In linear molecules, the bond angle is: (A) 90o (B) 104o (C) 180o (D) 120o.
50. Ethane undergoes mainly addition reactions because it is: (A) a gas (B) a hydrocarbon (C) unsaturated (D) easily polymerized.
ANSWERS:
1.
3. A
4.
5. C- an acid is said to be strong if it completely dissociates in solution.
6.
7.
8. D- nuclear reactions cannot be used to purify water.
9. D- NaCL crystallizes without water of crystallization.
10.
11. D- group seven element do not have low ionization energy.
12. C- cathode ray cannot be deflected by a negative plate since they are positively charged.
13. B- enthalpy of combustion
14. A- isomeric
15. B- unsaturated
16.
17.
18. B- destructive distillation
19. D- addition of water cannot increase the rate of reaction
20. C- allotropic change
21. D- oxochlorate (i) acid is not monobasic
22. D- collision theory
23. A- SO2 is reducing agent because it is oxidized in the reaction
24. A- chemical decomposition
25. B- Pb(NO3)2
26. C- brick-red precipitate is formed. NOTE: FOR TOLLENS SOLUTION, SILVER MIRROR IS OBSERVER
27. C- the ionic radii is less than the atomic radii.
28. C- monochloroethene.
29. B- ionization energy.
30. D- the activation energy is very high.
31. D- 8
32. A- cryolite (NaAlF3).
33. A- ethylpropanoate.
34. C- metal-electrons, electrolyte-ions.\
35. B- lactose
36. D- graphite
37. C- electrovalent compounds are not volatile at room temperature.
38. D- hydrogen and deuterium.
39. C- incomplete combustion.
40. B- an acidic oxide.
41. A- germicide
42.
43.
45. D- hydrogenation.
47.
48. D- double salt.
49. C- 180o
50. C- unsaturated.





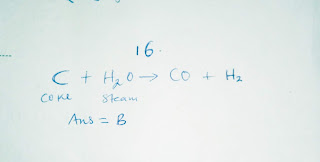





1 Comments
Right
ReplyDelete