Intramolecular Friedel-Crafts Reactions (with examples and illustrations)
Intramolecular Reactions
Here is an instant formula for an organic chemistry exam question.
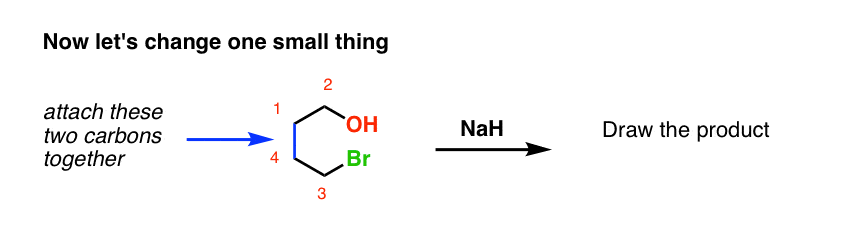
Start with a straightforward reaction that students understand fairly well, like the Williamson Ether synthesis….
(drawn weirdly, for a good purpose)
The second question above is an example of an intramolecular reaction, where the nucleophile and electrophile are on the same molecule, and the result of their reaction is that a ring is formed.
Why are intramolecular reactions good exam questions? Because they sort out the students who learn the reactions by memorizing a table of simple examples, and those who actually know (and most importantly, can apply!) the pattern of bonds formed and bonds broken. Furthermore, it involves no new concepts, which makes it totally fair game.
Intramolecular variants exist for a lot of different reactions, and it comes up so often that it’s worth mentioning separately. The Friedel-Crafts alkylation and Friedel-Crafts acylation reactions are no exception.
Intramolecular Friedel-Crafts Alkylation
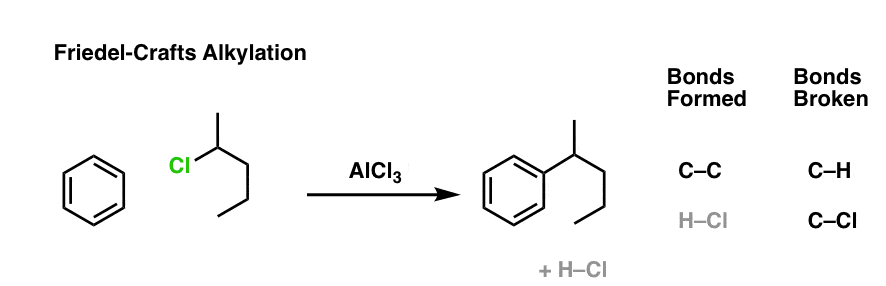
Here’s an example of an intermolecular Friedel-Crafts alkylation. The nucleophile is the aromatic ring, and the electrophile is the alkyl chloride. Add a little catalyst (AlCl3) and boom! electrophilic aromatic substitution.
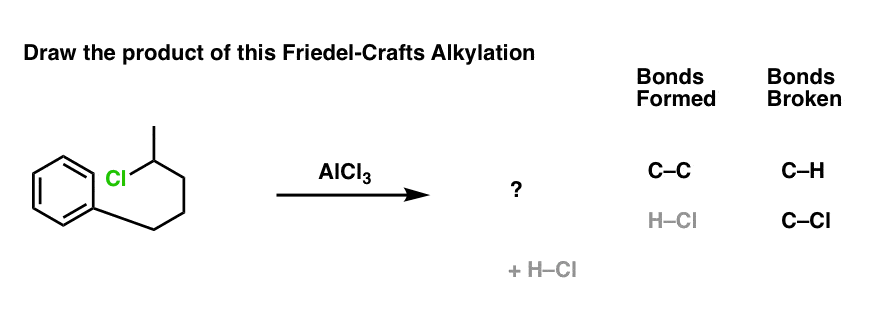
Now let’s change things up just a little bit. We’ll attach the alkyl halide to the ring via a new carbon-carbon bond, and then add the catalyst. What’s the product?
No new concepts! Same pattern of bonds that form and break. But if you haven’t seen an example like this before, it might throw you completely off your memorized notes.




0 Comments